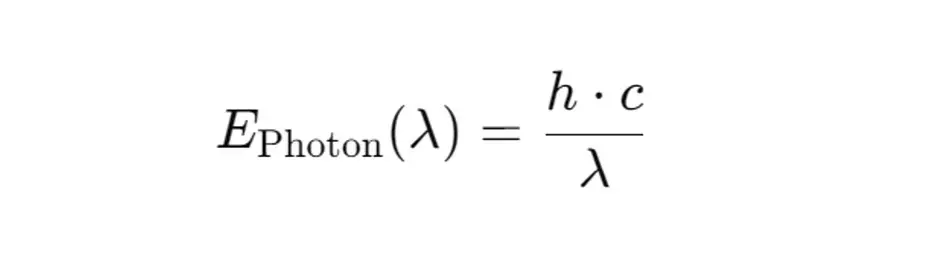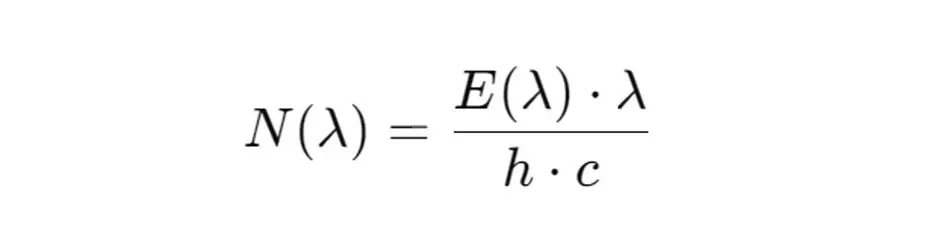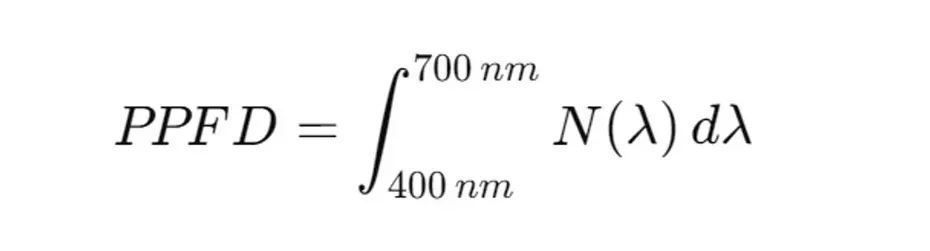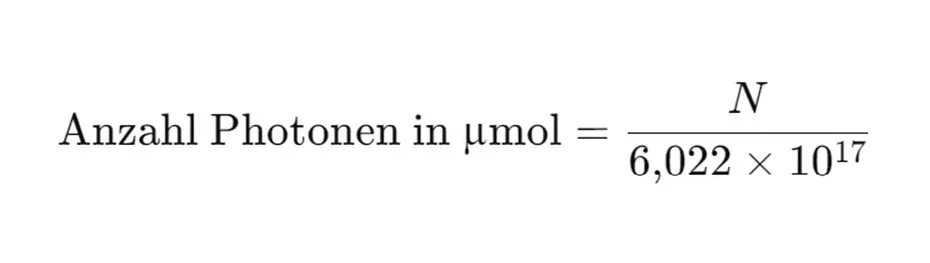Calculation of the photon flux density (PPFD)
The photon flux density (PPFD, Photosynthetic Photon Flux Density) describes the number of photosynthetically active photons (in the spectral range from 400 to 700 nm) that hit a surface per unit of time and area. It is given in the unit µmol-m-²-s-¹. This value thus provides information about the photon-based irradiance of a light spectrum, whereby only those photons are taken into account that can in principle contribute to photosynthesis.
In contrast to radiant flux density (watts per square meter), which describes the total energetic irradiance, PPFD is purely quantum-based. As photosynthetic processes are based on quantum mechanical excitations, the number of photons is more important than their total energy. This makes the PPFD a key parameter for assessing the effectiveness of light sources in plant physiology.
Application of PPFD in plant lighting
The PPFD is primarily used in the planning, optimization and control of artificial lighting systems in greenhouses, climate chambers and indoor farms. It is essential for:
- Determining the light intensity: Specific PPFD values are required depending on the plant species, stage of development and desired biomass production. For example, lettuce seedlings require approx. 100-200 µmol-m-²-s-¹, while flowering tomato plants can require up to 800 µmol-m-²-s-¹.
- Homogeneity test: The PPFD distribution on the cultivation area must be as even as possible to ensure uniform growth and avoid local over- or underexposure.
- Energy efficiency assessment: In combination with the power consumption of the lighting, the so-called "effective photon efficiency" (e.g. in µmol-J-¹) can be determined.
- Lighting control and automation: By measuring the PPFD in real time with suitable sensors, lighting systems can be dynamically adapted to the needs of the plants and external lighting conditions.
The calculation of the photon flux density (PPFD) from the spectral irradiance requires an integration of the spectral irradiance over the relevant wavelength range (typically 400 to 700 nm for photosynthesis) and the conversion of the energy units into photons. The spectral irradiance E(λ)E(\lambda)E(λ) indicates the distribution of the irradiance (W/m²/nm) as a function of the wavelength λ\lambdaλ.
The spectral irradiance is measured using an array spectroradiometer. This device uses a light-sensitive detector array that simultaneously detects spectrally resolved radiation via a spectrometer. Calibration is used for precise conversion into physical units of irradiance (W/m²-nm).
The basic steps for calculating the PPFD are as follows:
1. Calculation of photon energy
The energy of a photon at a certain wavelength λ can be calculated using Planck's formula:
This means:
- EPhoton(λ) is the energy of a photon at the wavelength λ in joules,
- h is the Planck constant (6.626×10-34 Js),
- c is the speed of light (3.0×108 m/),
λ the wavelength in meters.
2. Conversion of the spectral irradiance into the number of photons
The number of photons N(λ) that contribute to a certain spectral irradiance E(λ) at a wavelength λ can be calculated as follows:
This value indicates the number of photons per second and per square meter (µmol/m²/s) in the relevant wavelength range.
5. convert units
The resulting sum gives the PPFD in photons per square meter per second (photons/m²/s). To get from photons to µmol photons, divide by the Avogadro number (6.022 × 10²³ photons/mol).
To do this, the number of photons must be divided by 6.022×1017, as one micromole corresponds to one millionth of a mole: