Surface cleaning and surface activation with UVC and ozone
The cleaning and oxidation of optical and other components is of great importance in many high-tech areas, particularly in the semiconductor industry, optical manufacturing and aerospace. An efficient method for removing organic contaminants and improving surface quality is photochemical cleaning and oxidation using UV radiation at 185 nm in combination with ozone*.
This method uses the high energy of 185 nm UV radiation to generate ozone and reactive oxygen species to break down organic contaminants and clean the surfaces. The surfaces are also given a higher polar character, which has a positive effect on both the contact angle and the surface energy.
Areas of application:
- Cleaning lenses, prisms and optical filters
- Cleaning of wafers and microchips
- Removal of photoresist residues
- Preparation of substrates for coating processes
- The surface activation of bonded joints
- Removal of organic residues and fingerprints
- Sterilization and cleaning of medical devices and implants
Decoloration and odor removal
Basics of surface activation with UVC and ozone
Photochemical cleaning and oxidation is based on irradiating the components to be cleaned with UVC radiation at a wavelength of 185 nm. This wavelength is in the UV-C spectral range.
In direct photolysis, the 185 nm UV radiation splits covalent bonds in organic molecules. This leads to fragmentation and volatilization.
UV-C radiation with a wavelength of < 242 nm has sufficient energy to split oxygen molecules (O₂) into reactive oxygen atoms (O). For the UV generation of ozone, 185 nm and 172 nm are used in technical applications or ozone generators are used. 185 nm has a photon energy of 6.7 eV and is generated by ozone-generating low-pressure mercury lamps.
172 nm has a photon energy of 7.2 eV and is generated by xenon excimer lamps.
Ozone is produced by UV radiation with a wavelength of less than 242 nm as a result of the dissociation of molecular oxygen; most strongly as a result of radiation with a wavelength between 175 nm and 200 nm.
O2 +UV (λ < 242 nm) → 2 O·
The atomic oxygen radicals then react with other oxygen molecules to form ozone: O· + O2 + M → O3 + M (where M is a collision partner)
The ozone formed reacts highly effectively with organic impurities and oxidizes them to CO2 and H2O, for example.
In the presence of moisture, ozone can also lead to the formation of hydroxyl radicals, which also have a strong oxidizing effect:
O3 + H2 O + UV → 2 OH· + O2
O· + H2O →2 OH· (in water)
H2O + UV→OH· +H· (in water)
O3 + H2O + UV → O2 + H2O2 (in the gas phase)
OH radicals have a very high oxidation potential (2.8 V)
Hydrogen peroxide and titanium dioxide are used in water and wastewater treatment.
Hydrogen peroxide is split into two hydroxyl radicals by UV radiation:
H2O2 + hν → 2 ·OH
The addition of hydrogen peroxide is also based on the formation of hydroxyl radicals (·OH), which are among the strongest known oxidizing agents and are capable of breaking down a large number of organic and inorganic pollutants.
Titanium dioxide acts as a photocatalyst and is activated by UV (<280 nm), creating electron-hole pairs:
TiO2 + hν → e- + h+
These lead to the formation of reactive species such as ·OH and O·.
In general, the combination of UV radiation and ozone enables thorough cleaning and activation of surfaces. The UV radiation breaks down organic compounds, while the ozone further oxidizes them and converts them into gaseous products that can be easily removed from the surface.
Part of the UV radiation between wavelengths of 200 to 260 nm is energetic enough to split the carbon-carbon bond in the primary step, for example, or in the case of chlorinated hydrocarbons mainly the carbon-chlorine bond.
The following diagram shows the relationship between energy (E) in electron volts (eV) and wavelength (lambda) in nanometers (nm) for different chemical bonds.
The x-axis represents the wavelength from 100 nm to 400 nm, while the y-axis shows the energy from 0 eV to 14 eV.
The arrangement of the bonds on the curve shows that triple bonds such as N≡N and C≡C have the highest energy, followed by double bonds and finally single bonds. This corresponds to the decreasing bond strength from triple to double to single bonds.
The molecular binding energies can be found in the following table:
Molecular bonding | Binding energy (eV) | lamda (nm) |
N≡N | 9,8 | 126,5 |
C≡C | 8,7 | 142,5 |
C=C | 6,3 | 196,8 |
O=O | 5,1 | 243,1 |
O-H | 4,8 | 258,3 |
H-H | 4,5 | 275,6 |
C-H | 4,3 | 288,4 |
N-H | 4,0 | 310,0 |
C-C | 3,6 | 344,4 |
ADVANCED OXIDATION PROCESS (AOP) WITH UV AND OZONE
Ozone itself is a strong oxidizing agent and decomposes into molecular oxygen and reactive oxygen radicals when exposed to UV radiation at 254 nm or through thermal processes. These reactive species are highly effective in decomposing organic contaminants on the surfaces of components. This is why UV oxidation is also referred to as the Advanced Oxidation Process (AOP).
On the one hand, UV oxidation is based on the ability of UV radiation to break chemical bonds in molecules. When irradiated with UV-C, particularly in the vacuum UV range (172 nm and 185 nm), long-chain molecules are broken down by direct photolysis.
The advantages of this process include chemical-free cleaning, the avoidance of residues and the ability to treat even complex geometries. The method can also be carried out at room temperature, which makes it particularly gentle on sensitive optical components.
Air is mainly absorbed by its main components nitrogen and oxygen. The Schumann-Runge bands of molecular oxygen are particularly important for absorption in the ultraviolet (UV) range.
The Schumann-Runge bands are a series of absorption bands of the oxygen molecule in the ultraviolet spectral range between 175 and 204 nm.
172 nm and 185 nm therefore have a very strong absorption in air and therefore only a low penetration depth.
The UV absorption of ozone in the spectral range from 242 nm to 320 nm is known as the Hartley band and has a maximum at approx. 255 nm.
Typical penetration depths are
- 172 nm: Penetration depth approx. 0.1 cm to max. 3 mm
- 185 nm: Penetration depth approx. 10 - 20 mm
These values make it clear that UV radiation at these wavelengths is very strongly absorbed in the atmosphere and only reaches very short penetration depths.
AOP PROCESSING AND PROCESS OPTIMIZATION (UV & OZONE)
The dependence of the degree of absorption on the wavelength shows that the wavelength of UV radiation plays a major role in the oxidation process. Short-wave radiation shows a strong dependence on distance.
- Ozone is generated close to the UVC lamp.
- Ozone absorbs radiation with wavelengths of less than 300 nm and has a maximum absorption at around 255 nm - 260 nm.
- The lifetime of ozone molecules can be up to several minutes.
The process parameters are therefore
- Irradiation dose
- Power of the UV lamps
- Duration
- Distance
- Oxygen content / O -partial pressure2
The oxygen content in the ambient atmosphere plays a decisive role in the efficiency of the process:
Air has an oxygen content of 20.9%.
- Increased oxygen content:
- Increasing the oxygen content above 20.9% can increase ozone production.
- However, an excessively high O2 concentration can impair UV transmission and thus reduce direct photolysis.
- Reduced oxygen content:
- Ozone production decreases at oxygen concentrations below 20%.
- Direct photolysis becomes more important in this case.
- Controlled atmosphere:
In some applications, a controlled atmosphere with optimized O2 content can be used to maximize process efficiency. Oxygen levels of 1% are common at distances of a few cm.
Penetration depth and surface effect: The penetration depth of the 185 nm UV radiation and the ozone is limited, which makes the process primarily surface-oriented:
- UV radiation (185 nm):
- The penetration depth in air at normal pressure is only a few millimeters.
- In solid materials and liquids, the penetration depth is even lower, often in the nanometer range.
- Ozone:
- Ozone can penetrate deeper into materials than UV radiation.
- The penetration depth depends on the porosity and chemical composition of the material.
- In dense materials such as glass or metals, the effect is limited to the surface.
- Surface modification:
- The limited penetration depth leads to selective surface modification.
- This is advantageous for cleaning and activating surfaces without impairing the bulk properties.
- Geometric factors:
- The efficiency of the treatment depends heavily on the geometry of the parts to be cleaned.
- Complex shapes or undercuts can lead to shadowing effects.
To maximize the efficiency of photochemical cleaning and oxidation, the following parameters can be optimized:
- UV intensity and exposure time:
- Distance between UV lamp and treatment object
- Oxygen content and humidity
- Rotation or movement of the parts for uniform treatment of all surfaces.
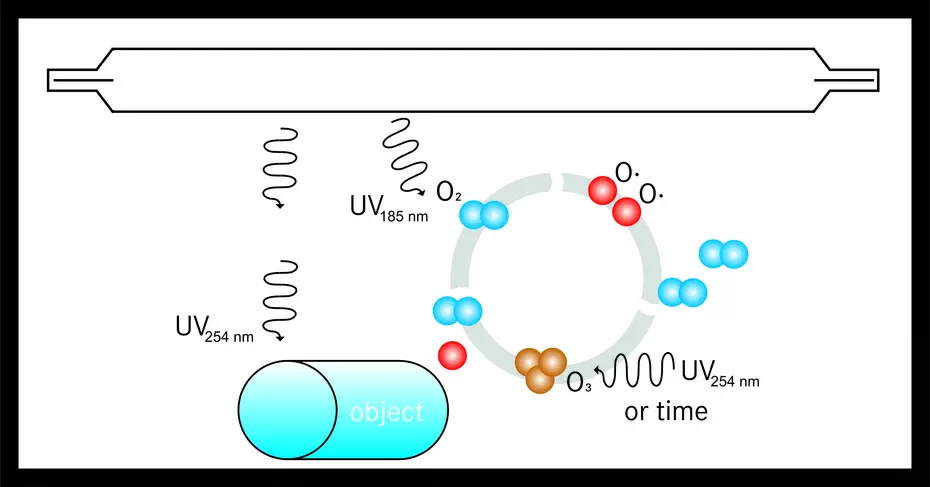
The following image shows the process of surface cleaning and surface activation with UVC and ozone and ozone formation in an irradiation chamber. The combination of direct UV irradiation and ozone formation enables effective surface cleaning and surface activation.
Summary and comparison of the advantages and disadvantages of UV oxidation
- UV oxidation offers a fast and efficient method of removing pollutants.
- It is possible to break down a wide range of organic and inorganic pollutants, including substances that are difficult to break down
- In contrast to chemical oxidation methods, no additional chemicals are introduced into the system.
- The process can be carried out at room temperature and atmospheric pressure.
- Better wettability due to higher surface energies
- No use of precursor chemicals required
- Possibility of on-site production
- High purity of the ozone produced
- Better wettability due to higher surface energies
- No use of precursor chemicals required
However, safety aspects must be taken into account during use, as both the UV radiation and the ozone produced can be harmful to health. Suitable shielding, ventilation and exhaust air treatment are therefore required.
UV oxidation in irradiation chambers
UV radiation creates ozone and oxidizing groups in the combustion chamber. Plastics and elastomers are attacked. The interior of the irradiation chambers should be as free of these materials as possible. The materials used must also be resistant to oxidizing substances.
Ozone leakage and extraction must be guaranteed.
Opsytec Dr. Gröbel builds customized ozone-generating irradiation chambers. We would be happy to discuss your requirements with you.
* Ozone is a very toxic gas in the right concentration . The generally recognized limit value is 0.1 ppm (parts per million). This corresponds to the MAK value (maximum workplace concentration) of 0.2 mg 03 per m3 of air. Due to its characteristic odor, it is perceptible even at this low concentration, so that those affected can take the necessary protective measures themselves, e.g. exhaust ventilation. However, the characteristic odor is only perceptible for a short time.
Ozone can cause damage to the lungs in particular. Concentrations of 1 to 2 ppm for several hours can cause headaches, chest pain, dryness and irritation of the upper respiratory tract. Even higher concentrations can lead to pulmonary edema with long-lasting health effects.